Rayonex Biomedical technology is the result of the life-long investigations of its founder, the German engineer Paul Schmidt, in the field of Biofeedback. Nowadays, it allows for a wide scope of applications whose effectiveness is supported by recent scientific trials both “in vitro” and “in vivo”. For example, cell cultures showed that cellular activity could be increased by up to 45% due to the effect of Bioresonance (study carried out at the Fraunhofer Institut). Several “in vivo” studies have been carried out so far; the most recently published one is a prospective, randomized, double-blind clinical trial in patients with neck pain presenting very positive results on its relief.
Rayonex’s quality control system according to standards DIN EN ISO 13485 (risk management for medical equipment) and regular audits done by a Notified Body ensure the quality consistency for these high-end medical devices. If you are a healthcare professional and want to include Bioresonance in your portfolio of services, these are the advantages offered by Rayonex Biomedical Bioresonance devices:
Showing all 2 results
 Rayocomp PS10 Med and accesories
Sorry, your country doesn't have access to view the price of this product.
Rayocomp PS10 Med and accesories
Sorry, your country doesn't have access to view the price of this product.
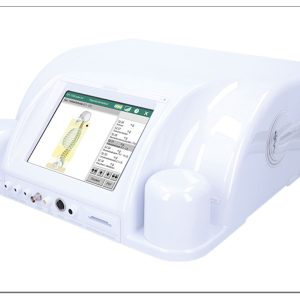 Rayocomp Polar 1000 4,0 Med and accessories
Sorry, your country doesn't have access to view the price of this product.
Rayocomp Polar 1000 4,0 Med and accessories
Sorry, your country doesn't have access to view the price of this product.
@2022 Geobionatura
